Stem Cells,
Growth Factors,
Targeted Specific
Bioelectric Signals.
Learn more
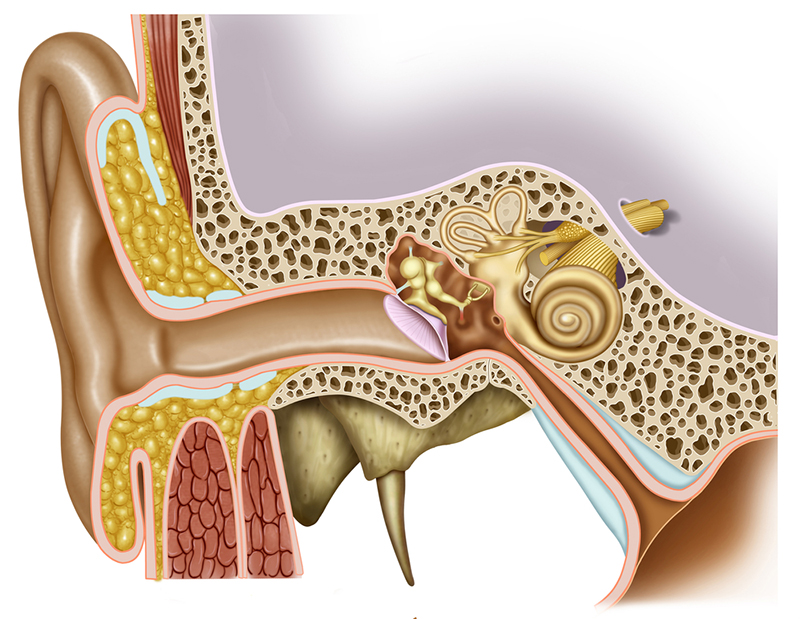
What if stem cells, growth factors and targeted specific bioelectric signals to control stem cell homing, proliferation, differentiation and protein expression could enable lost hearing to regenerate and heal?
50 Million
People in the US with some form of hearing loss
360 Million
People worldwide with some form of hearing loss
The company utilizes bioelectric stimulation to control release of specific proteins on demand for specific regenerative purposes in sequence:
2. VEGF (for new blood vessel growth).
3 IGF-1 (for DNA repair at the nucleus level).
4. Follistatin (for muscle and tissue regeneration).
5. RANKL (for demineralization and tissue loosening when needed).
6. Hepatocyte Growth Factor (tissue regeneration)
8. Tropoelastin (increases elasticity of any tissues such as skin, arteries, aorta, heart and promotes healing of wounds).
9. Activin B (regeneration).
10. GDF-10 and 11 (regeneration).
11. EGF – epidermal growth factor (regeneration).
12. FGF – fibroblast growth factor (regeneration).
In extreme cases we combine our regeneration microstimulator with an implantable, programmable, re-fillable stem cell/growth factor micro pump.
In cases of ear cancer we have special signals to stop tumor cell division and blood supply followed by the above regeneration protocol.
Klotho may prevent hearing loss
